http://crosleyautoclub.com/IcyBall/HomeBuilt/HomeBuilt.html
http://crosleyautoclub.com/IcyBall/HomeBuilt/HallPlans/IB_Directions.html

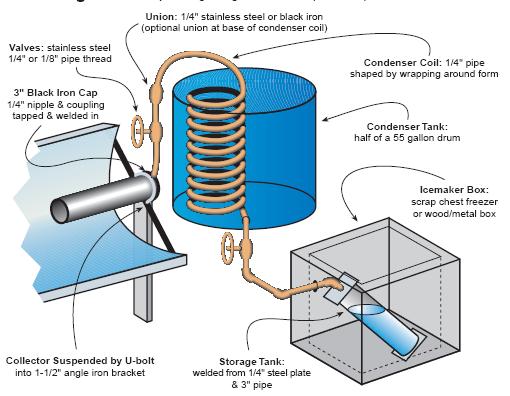
Water and ammonia combine easily. So, they combine at room temperature. Heat can then be applied to distill the Ammonia from the water and condense it in a separate container. The distilled Ammonia will then evaporate at less than room temperature and re-combine with the water. It is the evaporation of the Ammonia that then cools the container and the continued absorption of the evaporated Ammonia back into the water that allows the evaporation to continue until all of the distilled Ammonia is gone.
The obvious problem with this is that Ammonia is a terribly toxic chemical and any mechanical failure of the system which allows it to escape into an enclosed, inhabited space is likely to result in death. Zeolite / Water Adsorption systems, which work on more or less the same principle, are therefore much preferred.
See also:


Questions:
| file: /Techref/other/amoniafrig.htm, 2KB, , updated: 2013/5/15 17:19, local time: 2025/7/7 13:35,
216.73.216.200,10-1-86-173:LOG IN
|
| ©2025 These pages are served without commercial sponsorship. (No popup ads, etc...).Bandwidth abuse increases hosting cost forcing sponsorship or shutdown. This server aggressively defends against automated copying for any reason including offline viewing, duplication, etc... Please respect this requirement and DO NOT RIP THIS SITE. Questions? <A HREF="http://www.massmind.org/Techref/other/amoniafrig.htm"> Ammonia / Water Absorption Refrigeration</A> |
| Did you find what you needed? |
Welcome to massmind.org! |
|
The Backwoods Guide to Computer Lingo |
.